Pinched Mantle Syndrome in Giant Clams
By Dr. David Basti, Deborah Bouchard and Barry Neigut © 2009
Preface
As some of us are aware, Pinched Mantle Syndrome has been named by aquarist and was not clearly identified until approx. 6-7 years ago.
Clams Direct has been doing research for the past 5+ years and with the help of three labs and the most recent in Maine, we can now complied an official report of the findings that some of us suspected but nothing was documented until now.
Below is the final report that we received from Aquatic Animal Health Laboratory in Maine. We felt that it should be publish in the scientific term first. After reviewing the report, I will provide more information in easy to understand language .
We also want to thank all the people and some companies that donated to this research.
A healthy specimen of Tridacna maxima
Abstract
Pinched Mantle Syndrome is a term used by marine reef hobbyists to describe a disease condition in the ornamental clam Tridacna crocea and Tridacna maxima. The disease causes localized mantle retraction, gaping, loss of coloration and, eventually, mortality. A Perkinsus olseni-like protozoan has been identified as a possible cause of the disease. Based on this preliminary work, histopathology is the preferred method to confirm the presence of the parasite within the tissue of Tridacna crocea.
Introduction
Pinched Mantle Syndrome is a term used in the vernacular of the marine aquarium trade to describe a localized retraction of the mantle in the species Tridacna crocea and Tridacna maxima. It is a descriptive term and may not be pathopneumonic (pathogen-specific) for a disease syndrome that ultimately leads to the death of the clam if not treated. (More details regarding treatment, below)
The genus Tridacna contains 5 species of more or less commercial importance (Knop, 1996). All are found in the warm waters of the Indo-Pacific from east Africa to the Indian subcontinent, southeast Asia through Indonesia, northern Australia and the Great Barrier Reef, and into Polynesia. The IUCN (International Union for the Conservation of Nature and Natural Resources) lists them, depending on species, as lower risk to vulnerable. They have been used as a food source for millennia, and are aquacultured today for the Asian seafood market and for restocking depleted natural populations (Fatherree, 2006).
Perkinsus are protozoans in the newly- established phylum Perkinsozoa (Xue, 2006). They are closely related to the malaria and toxoplasma parasites of humans, as well as the photosynthetic-symbiotic Dinoflagellates found in the mantles of tridacnids and in the soft tissues of hermatypic corals.
The first species of Perkinsus was described in 1946 from a massive oyster die-out in Louisiana (Ray, 1996). Since then, 5 new species have been identified, the latest in 2004 in European oysters. Perkinsus olseni was first described in 1980 as a parasite in abalone colonies in south Australia and is detected in many bivalve species in tropical and subtropical waters (Goggin & Lester, 1995; Goggin & Lester, 1997).
There are three proposed stages in the life cycle of Perkinsus olseni (see diagram below). The trophozoite is the vegetative form that persists within the tissue of the host. It is thought that with the death of the host, and the development of anaerobic conditions within the host tissue, trophozoites enlarge into hypnospores (prezoosporangia). When the hypnospores are released into aerobic seawater they discharge high numbers of biflagellated zoospores, the infective stage of the parasite (Villalba et al, 2004; Auzoux-Bordenave et al, 1995).
Proposed life cycle of a Perkinsus olseni-like parasite (Auzoux-Bordenave et al., 1995; drawing by D. Basti)
The portal of entry for Perkinsus species is thought to be through ingestion. However, recent evidence indicates that the biflagellated zoospores can directly penetrate the gills and labial palps. Once inside the host, they are engulfed by hemocytes of the innate immune system, but are able to escape phagocytosis by inhibiting the cell’s respiratory burst of superoxide intermediates with the production of superoxide dismutase. They also elaborate perkinsin, an extracellular protease. They then multiply within hemocytes and are disseminated via the hemolymph to all the organs, and finally lyse and kill the host’s hemocytes. Death ensues due to tissue injury, the loss of innate immunity, and secondary opportunistic infections (Villalba et al, 2004). Clinical signs are severe emaciation, loss of body condition, gaping, mantle retraction due to the presence of trophozoites in connective tissue, retarded gonadal development, and mortality.
Example of Pinched Mantle Syndrome in Tridacna maxima; © Clams Direct
In the last 25 years there has been a growing worldwide niche market in the aquarium trade for the smaller, colorful tridacnid clams. Hobbyists are typically knowledgeable enthusiasts willing to invest time and money to maintain exotic species within reef microcosms. From an ecological perspective, it makes sense to develop technological and husbandry skills on a small scale, as a safeguard against the uncertain survival of wild species.
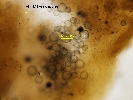
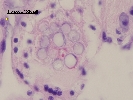
Hypnospores within gill tissue; © Clams Direct
The purpose of this study was to investigate the cause of Pinched Mantle Syndrome and to propose diagnostic and possible therapeutic options to reduce mortality in susceptible tridacnid species within the aquarium trade.
Materials and methods
Several shipments of Tridacna crocea were obtained by Barry Neigut, owner of Clams Direct, a retail distributor of ornamental clams in California.
Clams described as symptomatic for Pinched Mantle Syndrome and clams that were apparently normal were held in separate 40-liter glass aquaria. Each had its own independent biological filtration and foam fractionator. Artificial seawater (ASW) was prepared from “40 Fathoms” brand salt mix. The water temperature in each unit was kept at approximately 26 degrees-C, with a specific gravity (rf) of 1.025-1.026, and a salinity of 35 g/l. The pH was maintained at 8.0-8.2, alkalinity at 3.0 mEq/l. Ammonia (NH4+), nitrite (NO2-), and nitrate (NO3-) were kept at 0, less than 0.03, and 5-10 mg/l respectively. Water quality was maintained by a 25% water change (ASW) every 48 hours. Water loss due to evaporation was replaced with reverse-osmosis (RO) water. A 2-cm deep bed of aragonite sand was used to help buffer each system. Lighting was provided by a single 250-watt, 10,000˚ K (color temperature) metal halide fixture on a 10-hour daylight cycle. Clams were allowed to acclimate to the system for 10 days.
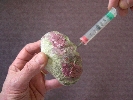
Aspirating hemolymph from Tridacna crocea; © Clams Direct
Non-lethal sampling was then conducted by aspirating 1.0 cc of hemolymph with a 3-cc syringe and a 23 gauge needle from the abductor muscle sinus of 13 clams. A 1-cm section of mantle tissue was surgically excised from 12 clams. Both samples were separately placed in Ray’s fluid thyoglycolate media (RFTM) with Streptomycin and Nystatin added to inhibit bacterial and fungal growth, and then incubated at 26 degrees-C for 7 days (Bushek, Ford & Allen, 1994). The clams were then allowed to recover. One clam succumbed 48 hours after the initial sampling, and tissue samples were submitted for histological preparation.
Gill tissue in RFTM at x40 magnification, with hypnospores visible as circular structures within the gill filaments; © Clams Direct
At the end of 7 days of incubation in RFTM, hemolyph samples and teased tissue samples were stained with Lugol’s iodine, which stains the thick-walled enlarged hypnospores a dark brown, which are then easily detected microscopically. Ten days after the nonlethal sampling, the remaining clams were sacrificed and whole body sections were submitted for histological preparation. In addition, RFTM tissue with hypnospores was submitted to MicroTechnologies Inc., Richmond, ME, for PCR confirmation and DNA sequencing.
Results
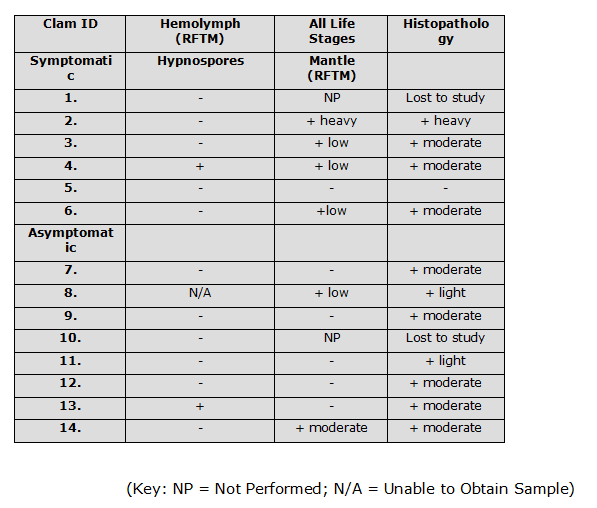
Table showing presence/absence of the parasite in symptomatic and asymptomatic clams. Note 1: unable to obtain sample; Note 2: not performed
Of the 13 hemolymph samples, hypnospores were detected in 1 clam symptomatic for disease, and in 1 apparently healthy clam.
Of the 12 non-lethally obtained mantle samples, 4 tested positive for hypnospores in the symptomatic group, and 2 tested positive in the apparently healthy group.
Histological examination of all the clams revealed the presence of the parasite in 4 of the symptomatic clams, and 7 of the apparently healthy group. One clam in the symptomatic group tested negative by non-lethal and lethal methods.
The DNA sequence of the isolated organism was found to be 95% homologous with the nucleotide sequence of Perkinsus olseni.
Discussion
Based on these preliminary results, low parasite burdens within the clams may be undetected using the non-lethal culture method of Ray’s fluid thyoglycolate media. A modification of the procedure to increase sensitivity may be necessary. It is reasonable to consider that a carrier state may exist in clinically normal clams and that other clams clinically symptomatic for Pinched Mantle Syndrome may be undergoing a different disease process. Although Tridacna crocea and Tridacna maxima are the majority within the trade to develop signs of Pinched Mantle Syndrome (Barry Neigut, pers. comm., 2007), Perkinsus species have been identified in 30 species of wild bivalves from the Great Barrier Reef. Among those of ornamental importance in addition to T. crocea and T. maxima are T. gigas, T. derasa, T. squamosa and Hippopus hippopus (Goggin & Lester 1987).
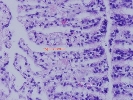
Hypnospores in gill tissue of Tridacna crocea; © Clams Direct
Marine reef hobbyists are advised to place their clams in a freshwater bath for 30 minutes (some species do better than others) if they demonstrate the symptoms of Pinched Mantle Syndrome(Barry Neigut, pers. comm.). It has been demonstrated that free prezoosporangia (hypnospores) are still viable after 6 hours in distilled water (Goggin, Sewell & Lester, 1990). The tissue-bound form of the parasite may also be protected within the host and capable of surviving chemotherapeutics that may either kill the clam, weaken the innate immune system, or pose a potential toxic threat to humans (Calvo & Burreson, 1994; Krantz, 1994).
Future work will continue to focus on correlating minimally invasive sampling techniques, such as needle aspirates of hemolymph or surgical samples of the mantle tissue, with the actual presence of the parasite. It may be necessary to develop more sophisticated serological tests with antibodies directed against Perkinsus-specific antigens or virulence factors.
It is important to culture the Perkinsus parasite in vitro, to subject it to antiprotozoal agents (LaPeyre, Faisal & Burreson, 1993). A method will then be developed to deliver the agent in vivo. This may take the form of a bath, micro-encapsulated drug, or a percutaneous injection. Any drug selective against Perkinsus may also have the undesired side effect of killing the symbiotic dinoflagellates within the clam’s mantle. The clams will then have to be repopulated with an exogenous source of the specific dinoflagellate.
The long term goal for the client should be to identify and avoid importation of clams from regional “hotspots” of the disease, and to market certified Perkinsus-free tridacnids within a biosecure facility that has a treatment protocol in place to clear infected clams of the parasite.
Practical considerations for hobbyists and retailers
Pinched Mantle Syndrome as we describe it in the hobby, is likely caused by Perkinsus olseni and possibly another unidentified species that parasitize a tremendous variety of mollusks throughout the world. The parasite probably originated in the Indo Pacific region and is now undergoing global expansion. It has been detected in Europe and most recently in South America. It is not clear exactly how it arrived in the western hemisphere, although it is possible that it is transferred through contaminated shellfish. It is considered a reportable disease by the World Organization for Animal Health (OIE).
The symptoms of Pinched Mantle Syndrome are caused by the presence of the parasite within the connective tissue of the mantle, causing irritation and inflammation and thus focal retraction of the mantle and signs of “pinching”. Have found the highest numbers of the parasite within the digestive system and not the gills or mantle, but in the end stages of the disease the parasite can be found in all tissues.
A specimen of Tridacna maxima exhibiting Pinched Mantle Syndrome; © Clams Direct
However, symptoms of Pinched Mantle Syndrome may also be caused by other disease processes that we have not identified, and by the same token clams can be infected that have absolutely no outward clinical signs of disease. Therefore the Perkinsus parasite may be brought into this country in clams that appear healthy. This is known as an asymptomatic carrier state.
At this time we have been unable to reliably and consistently detect the presence of the parasite without destroying the clam. This was one of the things that we were trying to develop.
It appears that Tridacna crocea and to a lesser extent Tridacna maxima are frequently infected, but we need to look at other species. It is our guess that others carry and succumb to the parasite based on surveys done in Australian reefs, but we haven't had the opportunity to look at T. gigas, T. squamosa, Hippopus hippopus or H. porcellanus. It is interesting that we have been unable to detect the parasite in Tridacna derasa, perhaps because we have not looked at enough samples but possibly because this species has natural resistance. If the latter, then this lovely species is clearly worth promoting.
We have a high index of suspicion and are concerned about Tridacna crocea originating from Vietnam. Alongside possible poor water quality issues in their holding lagoons, this parasite likely spreads rapidly in high density confinement.
We have not ruled out the possibility that this parasite could have an intermediate host. In other words this parasite could infect another type of mollusk and serve as a source of infection to tridacnids. This could easily happen in the wild or within a reef tank. Something else we want to look at.
The process of shipping and handling or placement of clams in less than ideal water conditions may result in stress that compromises the innate immune system and predisposes a clam that is carrying the parasite, to develop symptoms of Pinched Mantle Syndrome. It is also possible that there are many long term survivors of this disease being held in reef tanks throughout the world. Therefore the conscientious attention to water quality and habitat is very important. We observed confirmed positive Perkinsus-infected clams that appear quite normal and healthy when kept within relatively stress-free environments.
We would recommend quarantine of new arrivals for at least 2-3 weeks to observe for any obvious signs of Pinched Mantle Syndrome before placing them in the display tank. This is why we were so interested in developing a method to screen new arrivals without having to sacrifice them.
We would also recommend disinfecting your system with Clorox-brand (sodium hypochlorite 6.15%) at 1 tablespoon per gallon of water for 1 hour, then neutralizing with sodium thiosulfate. This should include all utensils and other implements used within your system. Be careful though because this is very toxic, and be sure to wear appropriate safety gear (such as gloves) and ensure good ventilation in the room where it is being used. Vinegar, although useful for removing calcium carbonate deposits and much less toxic, will likely have little effect on the parasite.
Fresh water baths of tridacnid clams may still be useful, but keep in mind that it will cause a certain amount of stress (more on this later).
Stress caused by things like excessive changes in temperature, salinity, lighting, fish nipping at the mantle, and so on will likely compromise a clam’s immune system and in turn make Pinched Mantle Syndrome more likely.
We believe that careful selection of clams, quarantine, and proper acclimation as well as research on the part of the fishkeeper prior to purchase are all essential to successful husbandry of Tridacna in home aquaria.
Treatment: fresh water dips
Place the clam in RO water that the temperature is the same as your tank and (ideally) adjust the pH to match that of the aquarium. Move the clam from side to side in the water, and this will help get some of the RO water in between the mantle and shell where the parasite seems to be present.
A specimen of Tridacna maxima 48 hours after an RO dip; © Clams Direct
Try to place you fresh water dip container under some lights to encourage the clam to open a little. Take a turkey baster and shoot water at wherever the pinch it. You can leave the clam in there for 20-30 minutes. Tridacna crocea may be exposed to even longer periods of immersion, as we found that they tolerate RO dips better than T. maxima. The clam can then be returned to the aquarium.
You may find that this process will have to be repeated several times, but we would suggest waiting a day or so between dips so that the clam can recover from the process.
Other things to consider
The protozoan can be dormant when the clam is happy and healthy, only for signs of Pinched Mantle Syndrome becoming visible when the clam is stressed. Stress can be triggered by many things, including shipping, poor acclimation, poor water quality, the wrong lighting, and bad choices of tankmates.
In many cases animals that are stressed have a weaker immune system, making them less likely to fend of infections, and consequently more likely to become sick or die. The classic example is something like Marine Ick (Cryptocaryon irritans) on a saltwater fish. The specimen may look perfectly healthy (i.e., asymptomatic) at your local fish store but a few days after it is purchased and placed in new environment, it suddenly exhibits the white spots symptomatic of the disease. In this case the fish was successfully holding back the disease while it was settled in the retailer’s tank, but once moved, its immune system was weakened by stress, and the fish was no longer able to keep the Ick infection in check.
Clams are likely similar in how their immune systems respond to stress, particularly when maintained in an aquarium. Analogies may be drawn with corals, particularly SPS corals. These often look great on the day of purchase, only to turn brown a few days later as they show signs of what we usually call Rapid Tissue Necrosis (or RTN).
And, to make matters worse, bacterial populations can be much larger in aquariums, as they are closed systems with relatively high nutrient levels. In fact, Fitt et al. (1992) wrote that even small amounts of organic material added to seawater can lead to a 1000-fold increase in bacterial populations within a matter of hours in some situations. -- James Fatherree, Advanced Aquarist’s
Online Magazine
On the other hand, not all pinched mantles are Pinched Mantle Syndrome. We have seen situations where a clam was stung by a coral, and the clam then pulls back the area of mantle where it was stung. That could be misdiagnosed as Pinched Mantle Syndrome, and the aquarist should keep an open mind when attempting to diagnose problems such as these.
In terms of treatment, some have tried using metronidazole. While this has helped, in some cases it kills the zooxanthellae. Because of this, we do not recommend that you use metronidazole in this way. We have also heard of hypersalinity being used to treat Pinched Mantle Syndrome. This can cause inflammation of the gills and prevents the shell from opening properly, causing the mantles to retract back into the shell and eventually cause death (Knop, 1996, Giant Clams). So again, this is not something we recommend.
With all the test that we have preformed, we have found that fresh water dip works the best and has by far the better survival rate.
So many hobbyists tell the same story: their clams were doing fine for awhile and then all of a sudden, they start to exhibit symptoms of Pinched Mantle Syndrome. It is very likely that in a lot of cases healthy clams carry some sort of protozoan infection, but don’t show any symptoms until something triggers them.
Summary
We know that shipping may cause stress to saltwater animals, not just tridacnids. We therefore recommend quarantining them after receiving the animal in a stress-free environment to help the clam recover and thereby making it better able to resist disease before it is placed it the display tank.
Newly delivered clams should be acclimated as quickly as possible after the shipment is received. The pH of seawater falls within a sealed bag while the enclosed animal respires. This means that the pH may be different in the shipping water to the water in your aquarium. Salinity may also be different. Make any water chemistry changes as slow as possible (e.g., by using a drip method) to avoid exposing the clam to undue stress.
When placing clam into tank, ensure that the lights are out. Again, this helps to avoid shock and stress. Remember, these animals have been in a dark box for up to 18 hours, and they are no less sensitive to sudden exposure to bright light as your fish. Also remember that different lighting systems will provide different levels of light intensity: your retailer may be using PC or T-5 lights, while your tank is equipped with MH lights.
Keep your clams away from any animals that could damage them. This obviously includes nippy fish or crustaceans, but do not overlook the fact that corals can reach out and sting nearby clams.
Close
It is our hope that further work will reveal safe and reliable treatments for Pinched Mantle Syndrome. Unfortunately, we have so far not been able to identify funds that could be used to investigate Pinched Mantle Syndrome and develop medications.
A healthy Tridacna maxima of the black variety; © Clams Direct
References
Auzoux-Bordenave, S., A.M. Vigario, F. Ruano, L. Domart-Coulon, and D. Doumenc, 1995. In vitro sporulation of the clam pathogen Perkinsus atlanticus (Apicomplexa, Perkinsea) under various environmental conditions. J. Shellfish Res. 14, 469-475.
Bushek, D., S.E. Ford, and S.K. Allen Jr., 1994. Evaluation of methods using Ray’s fluid thyoglycolate medium for diagnosis of Perkinsus marinus infection in the eastern oyster, Crassostrea virginica. Ann. Rev. Fish Dis. 4, 201-217.
Calvo, G.W. and E.M. Burreson, 1994. In vitro and in vivo effects of eight chemotherapeutants on the oyster parasite Perkinsus marinus (Mackin, Owen, and Collier). J. Shellfish Res. 13, 101-107.
Fatherree, James. Giant Clams in the Sea and the Aquarium. Tampa, FL: Liquid Medium Publications, 2006.
Ford, S.E., Z. Xu, and G. Debrosse, 2001. Use of filtration and UV irradiation to prevent infection by Haplosporidium nelsoni (MSX) and Perkinsus marinus (Dermo) in hatchery-reared larval and juvenile oysters. Aquaculture 194, 37-49.
Goggin, C.L. and R.J.G. Lester, 1987. Occurrence of Perkinsus species (Protozoa, Apicomplexa) in bivalves from the Great Barrier Reef. Dis. Aquat. Org. 3, 113- 117.
Goggin, C.L. and R.J.G. Lester, 1995. Perkinsus, a protistan parasite of abalone in Australia: a review. Mar. Freshwater Res. 46, 639-646.
Goggin, C.L., K.B. Sewell, and R.J.G. Lester, 1990. Tolerances of Perkinsus spp. (Protozoa, Apicomplexa) to temperature chlorine and salinity. J. Shellfish Res. 9, 1445-1448.
Knop, Daniel. Giant clams: A Comprehensive Guide to the Identification and Care of Tridacnid Clams. Ettlingen, Sweden: Dähne Verlag, 1996.
Krantz, G.E., 1994. Chemical inhibition of Perkinsus marinus in two in vitro culture systems. J. Shellfish Res. 13, 131-136.
LaPeyre, J.F., M. Faisal, and E.M. Burreson, 1993. In vitro propagation of the protozoan Perkinsus marinus, a pathogen of the eastern oyster, Crassostrea virginica. J. Eukaryot. Microbiol. 40, 304-310.
Ray, S.M., 1996. Historical perspective on Perkinsus marinus disease of oysters in the Gulf of Mexico. J. Shellfish Res. 15, 9-11.
Villalba, A., K.S. Reece, M. C. Ordas, S.M. Casas, and A. Figueras, 2004. Perkinsosis in molluscs: A review. Aquat. Living Resour. 17, 411-432.
Xue, Q.G., et al, 2006. A novel slow-tight binding serine protease inhibitor from eastern oyster (Crassostrea virginica) plasma inhibits perkinsin, the major extracellular protease of the oyster protozoan parasite Perkinsus marinus. Comp. Biochm. Physiol. B Biochem. Mol. Biol. 145, 16-26.
|
Giant Clams, family Tridacnidae on WWM Related Articles: Got Tridacna? A beginner's guide to keeping Tridacnid clams by Laurie Smith, Example Chapter from NMA Reef Invertebrates book, on Giant Clams, Tridacnids, A Brief Guide to the Selection and Placement of Tridacnid Clams by Barry Neigut, Bivalves, Mollusks, Lighting Marine Invertebrates, Related FAQs: Tridacnids 1, Tridacnids 2, Tridacnids 3, Tridacnid Clam Business, Tridacnid Identification, Tridacnid Behavior, Tridacnid Selection, Tridacnid Compatibility, Tridacnid Systems, Tridacnid Lighting, Tridacnid Placement, Tridacnid Feeding, Tridacnid Disease, Tridacnid Reproduction, Bivalves, Bivalves 2, Lighting Marine Invertebrates, |
|