|
FAQs about Faviid Coral Disease/Health, Pests 4
FAQs on Faviid Disease:
Faviid Disease 1,
Faviid Disease 2,
Faviid Disease 3,
Faviid Disease 5,
FAQs on Faviid Disease by Category:
Diagnosing,
Environmental (Pollution/Poisoning, Lighting...),
Nutritional, Social (Allelopathy),
Trauma,
Pathogenic (Infectious, Parasitic, Viral)
Predatory/Pest, Treatments
Related Articles: Coral Pests and Disease; pests, predators,
diseases and conditions by Sara Mavinkurve, Faviid Corals,
FAQs on Stony Coral Disease by Category: Diagnosing,
Environmental (Pollution/Poisoning, Lighting...),
Nutritional, Social (Allelopathy),
Trauma,
Pathogenic (Infectious, Parasitic, Viral)
Predatory/Pest,
Treatments
Faviids 1, Faviids
2, Faviids 3, Faviid Identification, Faviid Behavior, Faviid Compatibility, Faviid Selection, Faviid Systems, Faviid Feeding, Faviid Reproduction/Propagation,
|

|
|
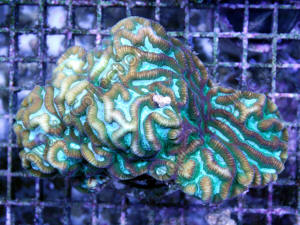
Maze Coral Question 8/14/10
<Hello Scott>
First off, I love your site, I have had so many of my common
questions answered by reading everyone else's experience.
<It's working then!>
Sadly I have a new problem that I am not sure I have been able to
find the answer to.
I recently bought this coral from my LFS, the attached picture is
straight from the LFS with my camera.
<Ok>
I have a 120 gallon tank that has been set up for a while now, I
recently placed this coral on a low lying rock at the bottom of
my tank. This morning when I woke up and was going to do a small
feeding to the various fish in my tank (maroon clown, Royal
Gramma, blue damsel, yellow watchman / diamond watchman
gobies)
<Please capitalize these names in future Scott>
I noticed that this coral kind of had a small layer of white
stringy substance all over it.
<Mesenterial filaments. If you look half way down this article
you can see a photo of a Hydnophora attacking a Montipora with
them. http://reefkeeping.com/issues/2006-07/gh/index.php
. I also seem to remember a great video of this with a similar
coral to yours on a David Attenborough programme, perhaps it was
'Blue Planet Coral Seas'
http://www.cosmolearning.com/documentaries/the-blue-planet-seas-of-life-405/19/
>
Some of my other corals, which were wide open when I went to bed
(Dendrophyllia, large very healthy hammer, frog spawn, torch
coral, Zoanthids)
<Again.. capitalize these next time please
http://www.wetwebmedia.com/wwmadminsubwebindex/question_page.htm>
were almost fully retracted when I woke up as well.
<Search re: Allelopathy, chemical/ physical interactions of
animals>
I did hours of research to try to find out about this white
stringy substance. It did not look like most of the feeding
filaments that I have read about, instead of being individual 1
inch strings projecting from the coral it seemed like a small
almost mesh-like layer of thing white strings over the coral.
<Could have been produced as an attack or defence mechanism,
or as a stress reaction to something. Either way, it has produced
a cascade of problems that need to be dealt with. First by water
changes, protein skimming & carbon use (perhaps ozone),
second by permanently separating some of the animals to different
systems>
I used a turkey baster and gently blasted most of the strings
away, and some of the larger pieces floated away which I guided
to my overflow filter.
<I would have netted these out>
Do you have any idea if these are problems or if I should do
anything about it?
<Posted. Immediate action required>
When I get home tonight I will inspect them again and try to take
some pictures.
<Yes! These would be appreciated!>
Thanks for your help
<No worries, Simon>
Re: Maze Coral Question 8/14/10
<Hi Scott>
Sorry for the poor grammar last email, I was in a rush and
slightly in a panic about my tank.
<No worries>
I did have a chance to talk to a LFS about my tank yesterday.
First some background info about my tank. about 2 weeks ago when
I was ready to start adding fish to my tank I was browsing the
LFS and saw a beautiful Blue Mandarin Dragonet. I asked the owner
if he could feed it so that I could tell if he would do well in
my tank. He dropped a small amount of Mysis Shrimp into the tank
and the Mandarin went crazy. I bought this Mandarin and he has
been doing great in my tank.
<What size is your tank? I would not place one of these in
anything less than a 90, with plenty of sand>
After about a week of him doing fine eating the Mysis Shrimp I
was feeding him, he started to eat less and less. He began doing
much more picking at the live rock for Copepods. I don't have
a separate refugium to breed a Copepod colony so I did some
research online.
<I would definitely look into setting a refugium up. Many
benefits to be had, especially if you have a Mandarin>
I found one aquarist who made a Copepod Condo out of some mesh,
zip-ties, and live rock rubble. He placed this directly into his
tank, the mesh was wide enough for the Copepods to go in and out
but too small for his Mandarin to have a buffet on the whole
colony.
<This sounds like a great idea!>
I decided to make one of my own. Long story short, I made the
Copepod Cage out of some steel mesh. Not thinking at all I placed
it in the main tank at about 10 pm on Wednesday night. When I
woke up in the morning I realized that metal should probably
never be in a fish tank. I removed it and then did a 40 gallon
water change that evening. I also added some Prime heavy-metal
remover into the tank.
<Mmm, did this co-incide with the event? If so, then it might
be the cause, but steel is actually Iron, Fe (with impurities
burned out and carbon added). It's not harmful in small
amounts and I seriously doubt that in one night this could have
contaminated your system. Some aquarists actually dose Iron. I
would not discount allelopathy here as the prime cause, perhaps
triggered by something else>.
This morning some of the corals are looking much better, the
Trachyphyllia is looking better, the Maze Coral has a
significantly less amount of the white stuff on it, but the
Dendrophyllia have not opened all the way, it is a 4 head colony
and since I did the water change the Dendro has had between 1-2
of the heads open, but never all of them open.
I am hoping that I caught it in time and that it will eventually
get back to normal now that the cage has been removed and that I
did a 1/3 water change.
Do you have any advice as to what I can do to get my tank back to
normal?
<I posted this on my last message, and my advice remains the
same. Water changes, carbon, skimming and separation of
incompatible animals>
I know this is one of those "oh my god, what did this idiot
do to his tank" situations, but I would love some help if
you know anything that I can do.
<Read here re: Iron & reef tanks: http://www.advancedaquarist.com/issues/aug2002/chem.htm>
Thanks again
<No problem, Simon>
|
 |
Dying moon ? 8/2/10
Hey fellas, just hoping to get some advice on my moon coral. I have had
it for over a year and have had very good luck keeping it nice and
healthy.
Just recently I have noticed the tissue has started receding and the
skeleton is pink where the coral has been degrading. My water
perimeters are monitored are just as they should be,
<Need actual values>
I run a calcium reactor, carbon, Purigen, and a high end skimmer so I
am positive water quality is not a factor.
<An essential material or two... could be missing. What do you feed
this colony? Supplement practice...>
I've just noticed there are a couple baby Aiptasia anemones on the
skeleton where death has occurred and am wondering if this could be the
cause.
<More an after-effect, though allelopathy may well be involved
here>
I also notice that I rarely see sweeper tentacles out at night like I
should. Could this be a problem with positioning, the coral is in
medium flow and in medium light (metal halide of course). My tank is
dominantly LPS and this is probably the easiest coral to keep. Is there
any quick remedy meds I can use,
<No... moving it elsewhere is recommended if you have another
established system>
I've read about Melafix for bacterial infections.
<No...>
How can I determine if it is a bacterial issue, I thought the pick on
the skeleton was a little fishy. Any advice would be great, this is one
of my favourite corals.
Thank you in advance
Jason K
Ontario, Canada
<Read here: http://wetwebmedia.com/cnidcompppt.htm
and the linked files in the series... Bob Fenner>
Re: Dying moon ? 8/2/10
Thanks for the advice and yea now that I think about it I bet you I do
have some chemical warfare going on. I have a few pretty large leather
corals, all sorts of mushrooms, pulsing xenia, 2 clams, and the rest
different
types of LPS, I don't think anything is touching
<Mmm, doesn't have to touch>
as far as that goes but that's why I've just recently started
running carbon and that Purigen stuff. I figured
if anything is going on this might help and maybe it has. I think the
degradation of this coral has stopped. And also I have recently added a
lawnmower blenny and it seems to love chomping on my one leather, maybe
it has been stressing it out enough to make it release some sort of
toxin.
<Possibly>
Another quick question. I know there is supposedly no such "reef
safe" Ich treatment. But I have found this stuff Ich-x
saltwater
<Have read re this Hikari "cure all"... am dubious>
and it claims to be totally reef safe. It says any stress induced will
recover. What do you think about all of this. I bought it a few months
ago because I have bought another hippo tang and just like every other
time I make this mistake, it ends up with Ich. It wont effect any other
fish in my tank but every hippo tang gets it in my tank lol. I'm
just a kid, I love my tank but I make some dumb mistakes. I seem to
think that if my tank seems to be free of it for like 6 months I can
just buy another blue hippo and it will be fine. But every time one
goes in this happens. I'm a sucker for this fish for some reason.
LOL. But back to the ICH-x, I probably wont try it because its
obviously ridiculous (unless you tell me different :) ). It says it
contains a proprietary formula of water and formaldehyde (<3%).
<?>
What do you think?
<Very toxic... and the chance for side reactions, disaster way too
great>
Chances are ill use my trusty blue hippo trap and get him into a
hospital.
BTW I have a 120 with only 2 clowns, the blenny, a marine Betta and
this tang. So I wouldn't consider it crowded.
Thanks again
YOU ROCK!!!
Jason K
Ontario, Canada
<And roll... I would read re Paracanthurus, Tang treatment/s for
Protozoans on WWM. B>
|
LPS -- 05/28/2010
I don't know if you guys can see the picture I am attaching,
if you can could you tell me if this candy coral is healthy
<It's alive. There appears to be some significant
recession of the tissue, but it looks generally ok.>
and also help me to identify the small shell looking creatures
taking over my rock.
<? I'm not sure I see what you're referring to,
sorry.>
I am worried because he still does not put out his feeders I have
only had him a couple of weeks. I know he was previously only
kept under one NO actinic light at the LFS for quite a while
before I bought him. My water parameter's are nitrate 0
nitrite 0 ammonia 0 ph 8.3 sg 1.025 temp 79 calcium 420 DKH 12.6
I also dose with magnesium and iodide when needed. I also do
weekly water changes adjusting calcium, DKH, ph, and sg to match
my tank. I only use RO/DI water for all purposes. I have these
under 124w of t5 HO 96w is 12k and 24w is actinic. My occupants
are 1 goby, 1 Percula clown, 1 coral banded shrimp, 1 arrow crab,
5 blue legged hermit crabs, 6 Astrea snails, 2 margarita snails,
2 Nassarius snails, and 1 peppermint shrimp. Everyone seems to
get along or at least they have until now, and I do plan on
getting rid of the arrow crab as soon as he finishes up the bulk
of my Fireworm problem.
<Fireworm problem? Not really any such thing... they're
beneficial. I wouldn't try to get rid of them.>
I have not seen any of the crabs or shrimp preying on or
bothering this coral and I have looked at all hours of the night.
Also I do run a Coralife 65g skimmer on this 29g system and
activated carbon. Any of your advice would be much appreciated
and used. Thank you again.
<How old is the tank? It's hard to keep corals in small
systems (no matter what you do)... especially if they're not
very well established.
Best,
Sara M.>
LPS, RMF's usual testy go 5/28/2010
I don't know if you guys can see the picture I am
attaching,
<Yes... even though it's much larger than what we ask
folks to send along...>
if you can could you tell me if this candy coral is healthy
<Is not... the algae, colour... >
and also help me to identify the small shell looking creatures
taking over my rock.
<The little white squiggles? They're Serpulids...>
I am worried because he still does not put out his feeders I have
only had him a couple of weeks. I know he was previously only
kept under one NO actinic light at the LFS for quite a while
before I bought him. My water parameter's are nitrate 0
nitrite 0 ammonia 0 ph 8.3 sg 1.025 temp 79 calcium 420 DKH
12.6
I also dose with magnesium and iodide when needed. I also do
weekly water changes adjusting calcium, DKH, ph, and sg to match
my tank. I only use RO/DI water for all purposes. I have these
under 124w of t5 HO 96w is 12k and 24w is actinic. My occupants
are 1 goby, 1 Percula clown, 1 coral banded shrimp, 1 arrow crab,
5 blue legged hermit crabs, 6 Astrea snails, 2 margarita snails,
2 Nassarius snails, and 1 peppermint shrimp. Everyone seems to
get along or at least they have until now, and I do plan on
getting rid of the arrow crab as soon as he finishes up the bulk
of my Fireworm problem.
I have not seen any of the crabs or shrimp preying on or
bothering this coral and I have looked at all hours of the night.
Also I do run a Coralife 65g skimmer on this 29g system and
activated carbon. Any of your advice would be much appreciated
and used. Thank you again.
<Have just skipped down. Read here:
http://wetwebmedia.com/FaviidDisF3.htm
and the linked files above, and re the Polychaete family
mentioned. Use the search tool/indices on WWM ahead of writing
us.
Bob Fenner>
|
 |
LPS questions, Faviid hlth. f' 5/23/10
Hello again, I recently bought a candy coral about three weeks ago
along with some mushrooms and a polyp I cannot identify. My mushrooms
and polyps are doing fine and starting to open up but my candy coral
has yet to
extend it's feeders.
<Likely mal-affected by the Corallimorphs>
My tank is a 29 gallon with 96w of t5 12k and 28w of actinics for the
lighting I run the actinics for 10 hours and the 12k for 8 hours the
actinics coming on an hour early and going off an hour later. I have a
Coralife super skimmer the 65 gallon model, I know you prefer a remora
so would I if I wasn't a college student and father of six. My
water
conditions are ammonia 0, nitrite 0, nitrate 5, ph 8.3, sg 1.025,
calcium 420, dKH 12, temp 79, and flow rate is around 550 gph the
majority of which is pointed away from the mushrooms and candy coral. I
do 5 gallon water changes every week and I also dose with iodide and
magnesium. The candy coral in question is about 14 inches from the
light maybe a little more. Am I giving him to little or too much light
is he still adjusting, I know many only come out at night but I have
come out with a flashlight at all hours and never seen even one polyp
open.
<Good observation>
I have however seen him secret the brown stringy mucus from his polyps
that is used algae correct?
<Possibly>
I do run carbon only through my canister filter and clean it every time
I do a water change.
<Good>
I also only use RO/DI water I have a four inch sand bed. I know these
animals are not completely photosynthetic and I worry that I have not
been able to feed him.
I have tried coaxing him out with a turkey baster with Mysis and with
brine shrimp. He seems healthy from what I can tell other than he does
not open he has 14 good sized polyps and it seems strange that not one
has opened. I
have however seen him swell up at night but no feeders any input would
be greatly appreciated. Sorry about all of the info on my tank but I
didn't know how much you need. Thank you
<Mmm, well... IF you had another established system, or a friend who
did, I'd move the Faviid... Do read here:
http://wetwebmedia.com/cnidcompppt.htm
and the linked "Compatibility" FAQs files re 'Shrooms
above. There are some other aspects of care that may be employed to
reduce probable allelopathy here. Bob Fenner>
Re: lps questions... Faviids, allelopathy f's
5/24/10
OK, thank you for the linked pages I think I may have messed up. I used
to have a bubble tip anemone but found a new home for it after learning
that it needs a larger system than the one I own. This anemone has been
gone for
about a month but the rock that was his home is the same rock that I
attached my candy coral frags to. I noticed before that all of the
algae on this rock had died and is slowly returning. After reading
about the toxins these animals produce in the linked pages I cant help
but wonder if there are still traces of these chemicals on and in this
piece of rock.
<Mmm, possibly>
As I said my mushrooms and polyps are doing very well and I got them at
the same time I got my candy coral.
<They "win" over Faviids chemically>
If my suspicions are correct other than using ozone is there any other
way I might be able to detoxify my system from this anemone.
<Carbon, water changes, time going by>
I don't want to do anything I am unsure of and any input would be
much appreciated. Thank you again for all of your assistance.
<Welcome. BobF>
Faviids Losing Tissue/Colored Skeletons --
05/17/10
Hey guys,
<<Paul>>
I have searched and searched and can't find any info on this issue
I am having.
<<Okay>>
I lost a Joker's Favia (purple and green) a few weeks ago and
basically the skin on the Favia just started to pull back from the
base, exposing the skeleton and in a week or so, was totally gone.
<<Mmm, most such events are related to water
chemistry/quality'¦in my experience>>
The skeleton was a bright red/pink color. Even after two weeks, the
skeleton stayed red/pink.
<<I have heard musings as to the reason why some Acropora
skeletons are 'green' at the time of tissue loss (infestation
of a boring alga species) which I suppose could apply to 'any'
hard coral species, but I have not heard about red/pink
skeletons'¦though if these theories are accurate, it could
be about any 'color' depending the alga involved'¦or
maybe it is the result from a microbial infection>>
Now it is happening to a Sponge Bob Favia (yellow and green). Both were
on the sand,
<<This may be adding to the problem if the sand is shifting and
causing tissue recession that might open avenues for bacterial
infection. Though many people do place these animals on sand, they are
generally not 'free living' organisms designed for such but
were likely broken from a hard substrate'¦placement on such
in the aquarium would be best, in my opinion>>
30" below the surface of my 300 gallon tank. The lights are 400W
MH with 12K ReefLux bulbs.
I have tried dipping the corals in Tropic Marin Coral cure with no
luck.
<<As long as any environmental issues causing the problem are not
identified and corrected, this would not help>>
Are Favia skeletons just red in color?
<<Have all been white, in my experience>><Are white...
though could be algal, bacterial coloring being added here once the
tissue is gone. RMF>
A few of my micros and Acans are receding as well but they are leaving
white skeleton behind.
My parameters are; temp 75.1*, pH 8.0 probe, 8.1,
<<'¦?>>
Seachem ammonia 0, nitrite 0, nitrate 30
<<High'¦ This very well may be the cause for the
tissue recession>>
(I feed heavy)
<<As do I, but you must employ means to keep Nitrate under
control (DSB, vegetable refugium, ancillary chemical filtration,
aggressive skimming, etc.)>>
KH 3.5 mEq/l or 9.8 dKH, Ca 440, Mg 1300, SG 1.024. I did a 50 gallon
water change on 12 May. Any ideas?
Paul Murphy
<<As stated'¦ There may be other contributing factors
(e.g. - placement), but based on your Nitrate reading I'm inclined
to think this is a matter of water quality. Cheers, EricR>>
Re: Faviids Losing Tissue/Colored Skeletons --
05/18/10
Eric,
<<Paul>>
Thanks for the reply.
<<Quite welcome>>
I tested water again today, with a new kit and nitrates are only 10ppm,
not 30 like the old kit was indicating.
<<Ah, a common issue'¦and much better>>
I will move the Sponge Bob up and hope it recovers. I dipped it again
yesterday.
<<I would still look at improving water
quality'¦perhaps adding some Poly-Filter chemical media to
the filter flow path. Cheers'¦ Eric Russell>>
|
Declining Caulastrea 5/4/10
Dear Bob and Crew,
<Howsit Joe?>
Again, the highest praise for your talents and time! WWM is a
blessing to all. Special thanks for the daily photos- they are
always surprisingly beautiful.
<Thank you>
I do hope this message is not too long. I felt it important to
include all relevant information.
<Please always do>
I'm in the middle of revamping my 54 gallon corner reef tank
after an outbreak of Ich. Luckily I only lost two fish and the
system has been fallow since 3-1-10. Only 3 corals exist as of
now here: 2 Euphyllias and
one Candy Cane, the latter which is partially receding (see pic).
The system utilizes a skimmer, 150 watt 14,000K HQI, 20 watt NO
actinic and is relatively low flow with about 700 gph turnover
rate from return, rotating powerhead, and HOT skimmer. I
downgraded this amount as I noticed better expansion from the
Euphyllias from lower flow. Today's water parameters are as
follows:
SG- 1.024 (normally kept at 1.025)
<I would keep this higher... check your hydrometer (if this is
what you're using to measure Spg) with a hydrometer>
Temperature- 78 degrees F
pH- 8.2 (a.m. reading)
DKH- 7
Ammonia- 0
Nitrite- 0
Nitrate- 0 (though present in tank)
<Good... your corals do need some>
Calcium- 350 (raising gradually with B-Ionic)
No magnesium test kit as of yet
<I would get, use>
The system uses Tropic Marin salt, Culligan RO water, and I have
not used any additives minus the B-Ionic as well as pre-buffer
for the RO water. I use Purigen in the sump. Specimens (not
including corals) include 2 Turbo snails, 3 Margarita snails, and
4 tiny hermit crabs.
The specimen is placed about 18 inches below the HQI bulb. My
best guess would be a lighting or water flow issue. I would guess
that the low calcium would not be the cause. I would also
discount allelopathy due to the few and like species involved. I
have not witnessed any hidden predation either. Any suggestions
would be highly valued.
Lastly, thank you so much for the new edition of "The
Conscientious Marine Aquarist"! My old copy was totally
destroyed from years of use and the new edition is a true
blessing!
Thanks!
Joe W.
<Mmm, let's see... I would move this colony up a bit
higher, on the rock, towards the light here... Please peruse
here:
http://wetwebmedia.com/faviidsysfaqs.htm
and the linked files above. Bob Fenner>
|
  |
Possible Parasite on Favia Frag... acting sans reading...
3/11/10
Hi,
<Hello Ben>
Thank you firstly for all the brilliant sound advice I glean regularly
from your excellent site, almost always if I have a problem, somebody
else has had the same and asked for help.
Almost always.
I have a Favia frag I recently purchased, however it at first seemed to
be doing fine attached to a rock down on my live sand bed.
<Most Faviids don't "live" on the sand>
But, I noticed recently a spot of dyeing
<And dying?>
flesh with bare stonework showing through, this is getting gradually
larger over the past 2/3 days and has now spread to 2 spots. I have
noticed that as well as it's normal feeder tentacles, the Favia has
much longer darker tentacles coning from underneath the rim, where the
Favia stops and the old skeleton/rock starts. Is this normal, or is it
likely that some kind of parasite has burrowed in and is sending out
tentacles to feed, as well as feeding off my Favia possibly?
<Can't tell from here; but whatever it/this is it not likely
parasitic>
Do you think the two are linked?
<Might be>
I have tried to kinda clean out round the rim of the Favia with
tweezers
<I would not do this>
(but to be honest I don't know if I'm doing more harm) and I
have used epoxy resin to seal all the bare rockwork so only the actual
Favia is now showing,
<Nor this>
again, is this correct or is it likely the 'thing' will now
either feed solely from my Favia, or burrow through it?
<... what thing?>
I have moved it to a different base and I will keep an eye on it to see
if this creeping disappearing flesh continues, any advice you have
would be most welcome.
<Read here: http://wetwebmedia.com/faviidae.htm
the linked FAQs file for the family, above... You haven't given any
data re water quality, other tankmates, the system, foods/feeding...
Nor a photo illustrating what you're getting at. Time for you to
read>
Finally, again, great work on the site, I think most of my corals/fish
have benefited from your experience at some point.
Ben.
<Please use it. Bob Fenner>
Neon Candy Cane Polyps/Health/Systems 2/15/10
Dear WWM Crew:
I bought this a week ago. The polyps have not opened since. I'm not
sure what to do to help the coral open up. It's close to some
Ricordeas & hairy mushrooms & a new cap coral.
Water param.s are CA 380, Alk 2.8 mEq/l, SG 1.024, Temp 78f, nitrate
2-3, Nitrites & Amm 0
<Not much useful system info present here so I do suggest reading
here and related articles/FAQ's re your Caulastrea.
http://www.wetwebmedia.com/faviidae.htm>
Thanks!
<You're welcome. James (Salty Dog)>
|
Sick coral - 2/10/10
Hi helpful and kind folks at WWM.
<Hello most wonderful and interesting Querior!>
I need your help with my favourite coral. <Ok>. I bought
this Favites from my LFS about 2 weeks ago (see picture labeled
1-29-10). Initially I had it at the bottom of my 6 gallon Nano
(36 watt PC lights) and it was doing well, so I moved it up about
4 inches to about the middle of the tank and glued it to a live
rock. A few days after that it was eating well during the day,
and extending some wonderful tentacles. Last few days it puts out
small tentacles at night only and refuses to eat when I try to
spot feed it Mysis (or anything else).
<Mmmm, they do feed mostly at night in the wild, I would not
be concerned re: this yet>
Most concerning is the discolored spot I noticed today (see other
pix) -- (? tissue recession)?
<No, I don't think so - maybe a small expulsion of some
Zooxanthellae, a reaction to being moved 'upwards'. Could
be an expansion of the coral itself. I would not be concerned too
much. Keep an eye out and see what happens, but this coral looks
ok to me. Monitor for a while, when it settles into it's new
spot it should be ok, these are not particularly difficult
corals>
Any help at all would be appreciated.
<You have my opinion, Nick. One thing I would say is that 6
gallons really is 'Nano' and if there is a problem with
the coral it is likely due to some part of your system being
unstable, as it is notoriously difficult to keep things 'on
an even keel' in such a small water volume, especially
temperature>
Nick N
|
 
  |
Re: 10/02/10 Sick coral
Thanks for your reply, Simon.
<No problem Nick>
As you can see from the attached picture, the discoloured area has
expanded from yesterday and (this may be hard to see in the
picture) the mouths in the affected area are becoming partly
black.
<Mmm, that does not sound good...did the onset of this problem
coincide with any change that you have made to the system, or in
the room where the system is housed?>
Any other ideas?
<Other than try to maintain some 'stable' parameters,
what other Cnidarian life do you have in here? Allelopathy could be
a cause, try here: http://www.wetwebmedia.com/cnidcompppt.htm. As
mentioned, in such a small system you will have problems with
creatures such as this. I would be running some carbon>
Anything else I should do? An iodine-based coral dip?
<Could, yes, this coral should stand up well to an iodine dip,
but I suspect that there is something in/ about your system that is
the root cause here. Simon> |

|
Re: 10/02/10 Sick coral
2/11/10
I'm very sad. The brown tissue was dead tissue.
<I would in that case dip this coral w/ Iodine
pronto>
I blew on the coral with a turkey baster and the brown
flesh flew off in chunks (surprising how much soft tissue
covers those hard skeletons).
<No! don't let this settle on any of your other
corals...if you must do this in tank it is better to suck
off with the baster and not blow>
I will probably frag whatever is still alive tomorrow.
<Mmm, be careful here... if you can stop the recession
w/ a dip then I would not frag this yet>
What a shame...
<Yes, indeed. Simon>
|
  |
Re: 10/02/10 Sick coral
2/11/10
<Hi Nick>
Thanks. No new livestock additions to the tank since this
coral was added. I do run Chemi-Pure Elite at all times as
well as Purigen. I have a small fuge in the back with Chaeto
which is growing like mad.
<This all sounds good>
The only significant change I have made recently is in FLOW.
I've added a Koralia Nano which is not pointing at the
coral, but toward the front of the tank. (see picture - yes,
I know about the Cyano and it is actually better as I've
cut back on feedings). I do know the Koralias do flow out
from the sides of the unit as well... The return from the
sump is also pointed toward the front glass of the tank, and
is on the opposite corner from the Koralia. I change 10 % of
the water twice each week. Could the flow be the problem?
<Only if it is directly on the coral, but the Koralias are
pretty good at negating these effects>
Should I move the Favites lower on the front rock to get it
farther away from the Koralia?
<If you think direct flow is a problem, yes>
The denizens of the tank currently are:
Two Montipora (different species)
Two Birdsnest
One Candycane Caulerpa
One small Oxypora frag (bottom right)
The purple fleshy coral mid-right was sold to me as a
Moseleya, though it doesn't look like it to me
A frag of a Dragon Favia (middle - front) which has turned
all purple, but is eating well (I will be upgrading my
lighting very soon)
Christmas Favia frag
A cluster of Blue Zoas at the bottom
A group of watermelon Palys at the top
<This is a Corallimorph?>
A head of an Acan lord at the very bottom left (this coral,
my very first, had been doing superbly for the past 3 months,
but over the past few days has suffered significant tissue
recession on one side, so I moved it farther away from the
Christmas Favia, which used to send out long sweepers)
A coral I do not know the name of (middle left, next to the
Koralia), which looks to me like a blunt-tipped Birdsnest
<Mmmm, you have a lot of different corals in here of
different types, especially for such a small volume. I have
less species than this in my system which is about 60 times
the water volume of yours. You will do better if you can
choose two or three species that you like, as similar to each
other as you can, and try to grow them larger>
A small fish (a Dottyback)
A few snails
Thanks again for your help,
<No problem Nick>
Nick
<Simon> |
|
Re: 10/02/10 Sick coral, Faviid
-- 02/14/10
<Hi Nick>
Thanks again - perhaps others can learn from my
mistakes.
<Some will, but these (putting many different animals in
a small water volume) are the most common mistakes
made>
Before receiving your response, I blew all of the dead
tissue off with the baster and the all of the pumps and
powerheads running - sounds stupid, yes, but my rationale
was that the chunks of tissue would end up in the sump and
I would just throw away the filter floss.
<Mmm, but this material is toxic...>
I was partly correct, though your way is obviously
better.
<Yes, but this is best done outside the aquarium>
I did not remove the coral to dip it, thinking to limit
stress on it.
<I would have dipped this as soon as I saw tissue
recession>
I did check my water parameters and they were all OK (0
Ammonia, Nitrite, Nitrate; pH 8.2; Alk 3.0meq/l; Ca: 440;
Phosphate undetectable; Mg 1260.) I then did a 15% water
change, changed the ChemiPure and Purigen, cleaned the
sump, and dosed a little iodide.
<Mmm, ok>
This morning the Favites and the Acan look a bit worse
overall, and both refuse to eat.
<I would remove this Favites now into another system if
you can, separate it from the others>
All of my other corals look good and the LPS eat Mysis like
it's going out of style. There is more Cyano today on
the sand (I had siphoned it all off yesterday).
<There is something amiss here..>
There are white spots on the border of many of the Favites
mouths - I noticed them a few days ago and thought they
might be feeding tentacles but they do not extend at all -
could they be a parasite? (see pic).
<No, not likely. More likely they are part of the coral
as you say>
My plan today was to dip the Favites and Acan in Tropic
Marin iodine dip and do another partial water change. What
do you think?
<I would go this route, maybe with separating these to
another system, and look to removing this/ these on a
permanent basis. I think your root cause here is too many
different organisms>
Nick
<Simon>
|
 |
Re: 10/02/10 Sick coral
2/24/10
Simon,
<Nick>
A quick follow-up and then a few questions: my tank is located
where I work, so after a long weekend I came back to find the
entire Favites dead - brown, smelly, much of the tissue receded
to visible skeleton. Horrible.
<Mmm, so you did not remove this as directed? Risky.>
Amazingly (especially considering the size of the tank (6 gal))
all other corals, and the fish, had survived.
<Amazing indeed, but I doubt they are thanking you for
it!>
Two of the three SPS were showing tissue recession from the base
up, so I thought they were goners. <Oh dear> I did a larger
than usual water change, fed only the fish for a week, and
everyone is looking good/better. The tissue loss of the Birdsnest
and Pocillopora has also stopped extending.
<That's a good sign at least. These would have gone in a
day had it been too bad>
Here is the question and the advice I need. I bought a Pinpoint
pH monitor, calibrated it and installed it yesterday.
<Mmm, not a fan of these...>
The pH in the tank was only 7.95! <You are actually ok here,
especially for such a small system>. Previously I was using
the colorimetric tests from API and Seachem, and I thought my pH
was in the 8.1-8.3 range (so hard to tell for sure with those
tests...). Did my usual 10% water change, and left the probe in
overnight, and the pH this morning was the same (I run a fuge
with reverse lighting). So I tested the source water, which I buy
premixed from the LFS - they state it's RO and that they use
Tropic Marin salt, and it's a reputable store so I believe
them. Well, lo and behold, the pH of the water I use to make my
water changes with is 8.00!
<Good, and fine>
So here is my question: should I add buffer to the water I use
for water changes before adding it to the tank to raise the pH to
8.3-8.4?
<What is your alkalinity? Never add 'buffer' unless
you know your alkalinity.>
If so, what should I use? I change 10% of the water twice a week,
and I dose Kent's CB to keep Alk. in 3-4 range (it rarely
gets above 3.5 even with dosing) and to keep Calcium in the
440-460 range (I have massive coralline growth all over the tank
:).
<Ahh, ok.. no I would not add anything here... it sounds as if
things are balanced and adding things to get to a 'magic
number' when everything else looks ok can do more harm than
good. The real clue is the animals themselves, and I am not a fan
of pH monitors. In my experience they can be inaccurate and need
calibrating constantly. What is your Mg?>
Or should I dose Kalk? ( I really would prefer not to since I
cannot set up drips at work and would actually prefer not to dose
anything daily). Or something else (I also own Salifert's
all-in-one but stopped using it because my alkalinity was staying
at 3)? What do you suggest?
<Nothing. Just monitor. 7.9 - 8.0 is fine here, really.
Especially since your colorimetric tests are reading higher than
this>
One other question-whenever I feed anything I have enriched with
Selcon (frozen Mysis or newly-hatched brine shrimp, for example),
I am guaranteed to have a nice new carpet of Cyano all over the
sand the next day. Is the HUFA supplementation that
important?
<This is definitely beneficial, and should not be a source for
Cyano, unless you are literally pouring the stuff in. Look
elsewhere for the answer to this problem>
I'm inclined to just feed the LPS's the occasional plain
frozen Mysis (shaken, not stirred), and a tiny bit of live
Roti-feast for the SPSs a couple of times per week in addition to
the fish poop they get (the Dottyback gets a few flakes each
day). Sound reasonable?
<Mmm, check here:
http://www.wetwebmedia.com/corlfdgfaqs.htm>
Thanks again for all of your help.
<No problem>
Nick
<Simon>
|
Unhappy Chalice Corals - Need Help -- 09/30/09
Dear WWM Crew,
<<Hello Laura>>
I have an 85 gallon reef tank with excellent water parameters
<<Real values please'¦>>
and one 150 HQI Metal halide bulb with 2 VHO 96 watt bulbs. I recently
ordered via mail (from a very reputable resource) two Echinopora
chalice corals.
<<Ah yes, a very popular coral'¦with some crazy
colors/color combinations to be found>>
They were beautiful upon arrival and I placed them mid level in my tank
on an open ledge of live rock,
<<Mmm'¦best to place these in a 'less bright'
location. Once acclimated to it they 'can' take the bright
lighting'¦but would still probably do/look better if
it's a bit more 'subdued' than what's received
mid-level in the tank under that MH bulb>>
where nothing could bother them.
<<Except maybe each other'¦ These are very aggressive
corals>>
This was two weeks ago, and they have been declining ever since.
<<Possibly photo-shock'¦or fighting amongst themselves
if placed to close together (this would be happening after tank
lights-out)>>
I am seeing tissue recession on the edges of the corals, as well as
both of them constantly struggling to "puff up" and then
deflate to show skeleton through their tissue. They look quite unhappy,
and I am very anxious to get them back to a healthy state. I feel they
are extremely 'stressed" and yesterday, I moved them to the
bottom of my tank under a live rock ledge that has lower, more filtered
lighting. I have been target feeding them with live phyto,
<<These are carnivorous animals>>
Cyclopeeze,
<<A good food choice here>>
and "Restore", a product for tissue regeneration containing
amino acids and HUFA's by Brightwell Aquatics.
<<Hmm'¦ Admittedly I'm not familiar with this
particular product from their line'¦ But I would suspect
direct supplementation with a proven quality food additive like
'Selcon' to be better money spent>>
The eyes open when I do this and they appear to take in food.
<<Absorption feeding is a possibility'¦but these
corals generally feed with the use of feeding tentacles'¦and
unless trained otherwise, is again an 'after lights-out'
event>>
They also appear to be expelling waste as a healthy animal would. As if
the stress of shipping were not enough for these poor animals, a
lighting change was made to my tank a few days into their arrival from
T5 lighting to the aforementioned MH and VHO set up.
<<Oooh'¦.likely part of the problem here>>
This was poor timing on my part, but the chalices were already showing
signs of being unhappy
<<But still'¦couldn't have helped>>
- primarily the recession and the puffing up and deflating I have
described. What else can I do to help these guys along and recover?
<<It's possible these corals were somehow malaffected before
you received them. These corals are fairly undemanding (once
acclimated). But'¦ Try placing them lower in the water
column and a bit to the side of the MH bulb 'and make sure
there are a few inches between them to preclude physical aggression.
Allelopathy is another possibility depending on your other livestock so
adding/beefing up carbon filtration (and/or Poly-Filter) may be of
benefit here>>
Also, will the tissue regrow over the edges that show recession if the
corals can be brought back to health?
<<Yes'¦ Should they recover, the corals will
regenerate that 'rolled-edge' look and grow over the old
skeleton much like any other hard substrate>>
I would appreciate any suggestions you can offer me.
<<I've made a few 'there's really not much
else you can do save move them to another system, to see if this makes
a difference>>
They are very beautiful and there is still a lot of healthy tissue on
them if they were to "bounce back."
<<If you can determine what is bothering these corals (too much
light 'too close together/too other aggressive corals) and
make a correction 'there's a good chance they can
recover>>
Thank you so much,
Laura
<<Happy to assist'¦ EricR>>
Re: Unhappy Chalice Corals - Need Help --
10/01/09
Dear Eric,
<<Hey Laura!>>
I apologize for the lack of water parameter information on my tank, and
I also noticed I forgot to include that I use a 14K Hamilton HQI MH
bulb in the lighting information.
<<It is best to give us as much info as possible (thank you for
this). You never know where a 'clue' might be
hiding>>
Water parameters are:
Ca: 450
dKH: 10
Temp: 78F
Phosphates: Undetectable
Nitrate: 0
Ammonia: 0
Magnesium: 1395
Salinity: 1.024
pH: 8.1-8.2
<<And agreed, no real problems here'¦ though I would
let either the Calcium or Alkalinity fall just a bit. Have you read
this? http://www.wetwebmedia.com/calcalkmar.htm>>
I do run carbon on the tank.
<<Excellent>>
The chalices do have several inches between them, the closest animal to
one being a crocea clam on the sand bed of the tank, now that I have
moved them to lower positions. This clam is several inches from the
chalice.
<<Okay'¦ But do try to observe the corals at different
times after the tank has been dark to make sure they are 'far
enough' apart. You might be surprised at their
'reach'>>
This morning the chalices appear to be "stable". Although I
am no expert,
<<Me neither! [grin]>>
I am suspecting lighting to be my biggest issue in their state of
health.
<<I too think that to be a contributing element here>>
Just out of curiosity, how long in the more subdued lighting would they
need to reside before I would see marked improvement if, this is
indeed, their issue?
<<Hmmm'¦ Based on your previous statements re, I would
expect to see improvement in as little as a week (flesh filling
out/expanding, color returning)'¦though it may take a while
(several weeks or more) to notice any 'significant' regrowth
over the damaged margins>>
This will be their second day in the dimmer lit area. I also have
Selcon and can begin using that product since it seems to be a proven
enrichment product for captive animals.
<<Indeed'¦ Do also look in to/get some New Life
Spectrum pelleted food. Aside from the benefits of this excellent
prepared food to your fishes, consider that it can also be fed to your
Chalice corals 'as well as Faviids, Euphyllids, and others
with similar feeding structures/tentacles/methods. I find the 1mm
pellets to work well all around>>
Thank you so much for all of your help!
Laura
<<Always happy to share'¦ Eric Russell>>
|
Sick Caulastrea 02/15/09 Hi All: Two months
ago, all of the corallites on this Caulastrea had polyps, which
expanded. Over past two months, most of them have died and the
few that are left are shriveled. During that same time the
tentacles which can be seen in the photo have been growing. The
tips are the same mint green as the polyps and they all emanate
from the same place on one of the stalks. They expand during the
day and contract at night. They are not feeding tentacles since
the feeding tentacles have white tips and emanate from the mouths
of the polyps. Do you have an idea what these tentacles are and
why this colony is dying? <Hmm... I do think this is an
anemone of some sort. But I can't ID it much further than
that from this pic. If it is an anemone, that could easily
explain why the trumpet coral is dying.> Thanks,
RB
<Cheers,
Sara M.>
|
 Mmm,
a Fungiid or Euphyllia is my guess. RMF Mmm,
a Fungiid or Euphyllia is my guess. RMF |
|
Re: Sick Caulastrea 2/16/08 Hi Sara: Thanks
for your reply. A couple of the readers of a thread on Reef
Central think it is a torch coral. Looks like one but I
haven't had a torch or anemone in my tank. Since the
Caulastrea was in the tank for 9 months before the
"tentacles" showed up I doubt that it was a hitchhiker.
What do you think? - RB <I had this thought as well (that it
looked like a Torch coral). But if it were a Torch coral (a
Euphylliid), you should be able to find its skeleton. -Sara
M.>
Re: Sick Caulastrea 02/16/09 Hi Sara: Will
you please have a look at the attached photo. The critter in
question is attached to the Caulastrea skeleton by the light
colored foot which is visible under the two corallites in the
center. I can't detect a skeleton, but could that be because
it is immature? Thanks - RB <Hmm... it still looks like an
Anemone to me, but Bob noted on your last email that he thinks
it's a Euphylliid... so... hmm... I suppose time will tell.
Cheers,
Sara M.>
|
|
|

